What Is Chronic Obstructive Pulmonary Disease (COPD)?
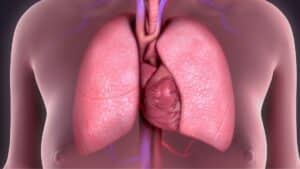
Chronic obstructive pulmonary disease (COPD) is a progressive inflammatory lung disease that causes difficulty breathing, as well as a range of other symptoms.
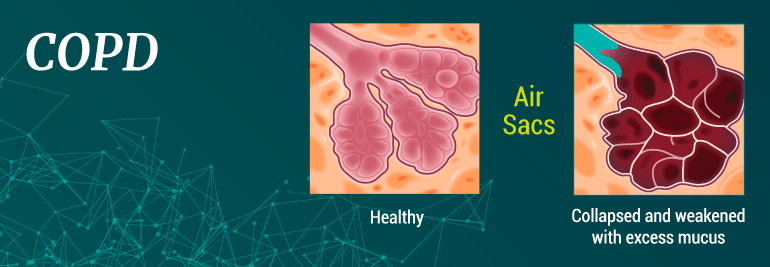
This condition is characterized by a restriction of airflow into and out of the lungs. Many people who have been diagnosed with this condition experience the sensation of not being able to catch their breath or have the energy to move around the house.
Many inflammatory lung conditions can fall under the umbrella of COPD, such as emphysema and chronic bronchitis. If you or a loved one has been diagnosed with or suspects COPD, take a moment to read through the information in this article and our website. Our goal is to help you Breathe Easier.
Symptoms of COPD
For many people, the symptoms of COPD are the first indicator of the condition. In some cases, patients may life with the symptoms for many years before scheduling an appointment with their doctor for an official diagnosis.
The most common symptoms associated with chronic obstructive pulmonary disease (COPD) include:
- Chronic cough
- Shortness of breath
- Frequent respiratory infections, sometimes that lead to hospitalization
- Blueness of the lips or fingernail beds from lack of oxygen
- Chronic fatigue
- Excessive mucus
- Wheezing
Often, people who are diagnosed with COPD have difficulty performing daily activities due to the lack of oxygen being receiving into their bloodstream. Activities such as going to the store or folding laundry cannot be completed without excessive resting between tasks so patients can catch their breath.
If you are experiencing any (or all) of these symptoms, you should schedule an appointment with your doctor to determine if you have COPD. There are treatment options available that may help you cope with these symptoms.
Causes of COPD
There are several factors that can contribute to the development of COPD, such as smoking, chemical exposure and genetics. Of these factors, cigarette smokers are at the highest risk of developing COPD. The causes of COPD are as follows:
- Smoking – The toxins in cigarette smoke absorb into the bronchial walls, causing inflammation and damage that leads to COPD. However, not all smokers develop symptoms. More research needs to be done as to why COPD only affects some smokers and not others. It is speculated that nutrition, genetic factors and other external influences contribute to the likelihood of COPD development.
- Genetics – Alpha-1-antitypsin deficiency causes COPD in a small percentage of people. Those with this deficiency do not need to be a smoker to be susceptible to this disease. It can also strike at a young age.
- Age – COPD is a progressive disease and therefore affects people later in life. As a result, current or former smokers 40 years or older are the most commonly afflicted with COPD. However, the age of the sufferer directly relates to the age at which his or her lungs were damaged. For example, a child who grew up in a heavily polluted area may be at risk to developing COPD much earlier in life.
- Chemical exposure – A long-term exposure to dust and chemicals can cause COPD-related inflammation in the lungs.
If you are experiencing COPD symptoms and you fall into any of these categories listed above, your next step is to schedule an appointment with your doctor to receive a diagnosis.
Diagnosing COPD
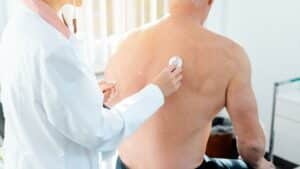
When you schedule an appointment with your doctor to determine if you have COPD, you can expect to undergo a couple tests before receiving a diagnosis. These tests are very simple, and often include a conversation about your family and medical history, lifestyle habits and current symptoms.
After this conversation, your doctor will likely have you perform a spirometry test. A spirometry test is relatively simple, and requires you to blow air into a tube that measures the amount of air coming out of your lungs. This will help your doctor determine your lung capacity.
Additionally, you may undergo a physical exam and some lab work to confirm a COPD diagnosis and to accurately determine the progressive stage of COPD that you have.
Treatment for COPD
Though there is no cure for COPD, there are many treatment options available to help you cope with the symptoms of this condition. Doctors typically prescribe the following treatments for COPD patients:
- Medication, typically steroids to reduce inflammation and help open the airways
- Inhalers
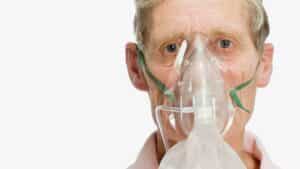
- Oxygen therapy
- Cellular Therapy
These treatments aim to relieve symptoms when they appear, but they do not aim to prevent symptoms from occuring. However, cellular therapy from the Lung Institute targets the root cause of COPD symptoms so patients can breathe easier and regain their quality of life.